Water in Brewing – The Full Guide
Many brewers are familiar with the three essential ingredients in beer brewing: malt, yeast, and hops. But what about water in brewing? We all know beer has water, and plenty of it, but we often overlook it.
Quick fact:
Throughout history, in areas where drinking water was not safe, beer served as a reliable and secure alternative. In fact, it was often considered a life-saving beverage.
Follow us on Instagram!
Water plays a crucial role, constituting more than 90% of your beer (the same goes for ginger beer and root beer). It is often said that the chemistry of your brewing water and how well it complements your beer can be the difference between a great beer and an exceptional one. You heard it right – water can make or break your beer. In fact, high-end brewers always focus on getting the best drinking water possible for their brews. Virtually all high-end brewers source their water from deep wells and aquifers (some brewers take great pride in having their own pure water source).

This article will guide you through the intricacies of water chemistry in beer brewing. We will explore various water sources, examine the different ions and chemical compounds found in water, and discuss their impact on the final product. Although water may initially seem like a simple ingredient, it’s made of complex chemicals such as salts, ions, and minerals that can be analyzed and adjusted during brewing. Yes, water is not just a simple molecule of one oxygen and two hydrogen atoms in a covalent bond. It’s much, much more…
A general rule of thumb for beginner brewers is that if your water is drinkable and has a pleasant taste (that means NO taste), it is suitable for brewing. However, it is important to keep in mind that certain water sources may have specific properties.
The Chemical Composition of Water – What You Need To Know
Do you want to make great beer? If yes, you have to delve into the chemistry and composition of your brewing water. And there’s a lot to know. But don’t worry; we’ll explain everything!
Key Water in Brewing Factors To Consider
#1 – The pH Levels
During the brewing process, pH levels exert a significant influence and must fall within specific ranges to optimize the brewing process. The water’s pH levels should be between 6.5 to 8.5. However, the main thing to focus on is the mash’s pH:
- During the mash, the pH should ideally range from 5.2 to 5.6. This range facilitates optimal enzyme activity in the mash.
- As the boil commences, the pH slightly decreases due to sugar reactions and the addition of hops.
- During fermentation, as yeast produces alcohol, the pH of the wort further decreases to approximately 4-4.5. This lower pH helps safeguard the beer against spoilage organisms. Note: For sour beers, the pH can dip as low as 2 in extremely sour varieties.
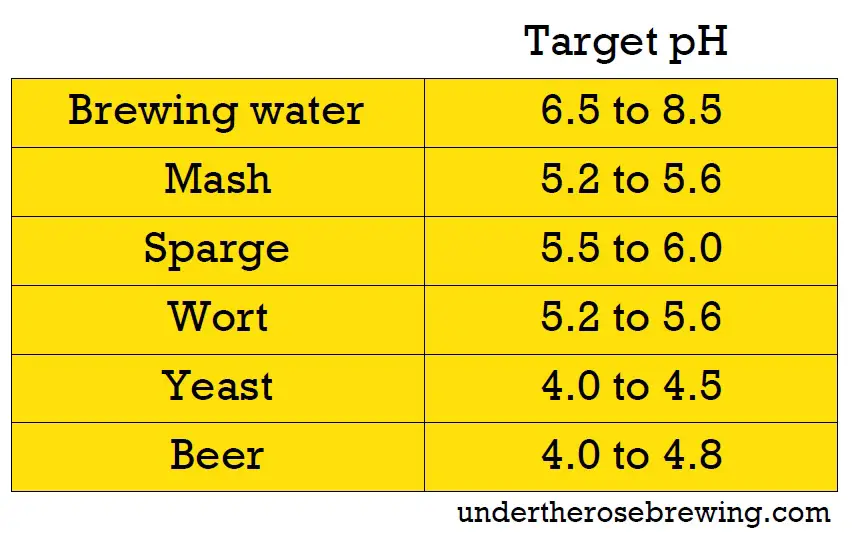
pH adjustments can be made at any stage of the brewing process using acids, bases, buffers, or salts. To calculate pH adjustments accurately, use the Grainfather water calculator. It factors in the pH of the source water, the type of grain used in your beer, and the specific style of beer you’re brewing.
#2 – Water Ions
Once the pH is addressed, it’s important to examine the presence of various ions in your brewing water. The levels of these ions will vary depending on your water source, and each ion contributes to the final taste of your beer, either directly or by influencing the perception of maltiness or hop character.
Ions are atoms (or groups of atoms) which carry a positive or negative charge caused by the loss or gain of electrons. In beer water, ions refer to the positively charged cations and negatively charged anions that result from the dissolution of minerals. The key cations we focus on are calcium, sodium, and magnesium. The primary anions include chloride, bicarbonate (together with carbon dioxide), and sulfate.
Calcium is the dominant cation that determines water hardness. Calcium makes beer more stable and clear, while increasing its flavor. Magnesium is also important to water hardness, but has a lesser impact on mash pH compared to calcium. Sodium, on the other hand, does not significantly make water harder. In very small quantities (<100 ppm), sodium is virtually harmless, but higher concentrations can impart a mineral or metallic taste to the beer.
Quick fact: Hard water, which contains higher levels of calcium and magnesium, tends to make darker, richer beers. On the other hand, softer water, with lower mineral content, gets you cleaner and more bitter ales.
Carbonate ions play a significant role in determining the chemistry of brewing water. Carbonate, as well as bicarbonate ions influence the overall alkalinity of the brewing water, raising the pH of both the mash and the beer. On the other hand, sulfate ions enhance hop bitterness, creating a drier and crisper taste. While sulfate ions are weakly alkaline, they do not contribute to total alkalinity.
In contrast, chloride ions give the beer a sweet, fuller flavor. They have the opposite impact of sulfate ions. The ratio of sulfate to chloride can provide insights into the balance of the beer influenced by the brewing water. For instance, a higher sulfate-to-chloride ratio of 2 to1 or more will result in a beer with a drier and more pronounced hop character. Conversely, a ratio of 1 to 2 will contribute to a beer with less bitterness, a rounder mouthfeel, and a maltier balance. It’s important to note that while the sulfate-to-chloride ratio can influence beer balance, it cannot fix a flawed recipe. It acts more like seasoning your food, accentuating existing flavors.
Lastly, you have to understand the difference between chloride and chlorine. Chloride is an anion in brewing water that affects taste, while chlorine is a disinfectant used in water treatment. The concentrations of both chloride and chlorine to each other are not relevant in brewing water.
Calcium plays a crucial role in brewing water, even though it does not contribute much to the flavor. Its importance lies in the following aspects:
- Increasing mash acidity: If you have highly alkaline water, calcium can help in lowering the pH during the mashing process.
- Assisting enzyme activity: Calcium supports the enzymatic functions during the mashing process.
- Extracting hop bitterness: Calcium aids in extracting the desired bitterness from hops.
- Reducing haze: Calcium helps in reducing haze formation in the beer.
- Decreasing wort color: It can contribute to a lighter color in the wort.
Common additions for calcium include calcium sulfate (gypsum) or calcium chloride. In the past, calcium carbonate (chalk) was used, but it is less soluble in water and has less impact compared to gypsum and calcium chloride.
#3 – Magnesium
Magnesium is important for promoting proper enzyme activity during mashing and acts as a nutrient for yeast. In small quantities, magnesium can enhance the overall flavor of the finished beer.
#4 – Sulfates and Chlorides
The ratio of chloride to sulfate is a crucial consideration for every beer style as it determines the beer’s balance.

Chlorides contribute to the flavor and perception of fullness in the palate, which is particularly desirable for malt-forward beers. They enhance sweetness and mellowness while also improving beer stability and clarity over time.
Sulfates are particularly significant in hoppy beers, as they enhance hop character, aroma, and the perception of bitterness.
The balance between chloride and sulfate ratios determines the overall character of the beer. If sulfates are proportionately higher, the beer will be hop-forward. Conversely, if chlorides are proportionately higher, the beer will be malt-forward. Equal levels of chloride and sulfate create a balanced beer.
#5 – Sodium
Sodium can be used to add alkalinity to brewing water and can improve the flavor and mouthfeel of the beer. However, excessive levels of sodium can result in a harsh, salty taste and can be harmful to yeast. Care should be taken to maintain proper levels.
#6 – Hardness and alkalinity
Water hardness refers to the concentration of dissolved calcium and magnesium in the water. Hard water contains higher levels of calcium and magnesium, while soft water has lower concentrations of these minerals. Water softeners function by chemically replacing calcium and magnesium with sodium or potassium.
However, this poses a challenge for brewers. As mentioned earlier, ideal brewing water should have moderate hardness. It should contain a minimum level of total hardness of around 150 ppm of calcium carbonate (CaCO3). Water softeners effectively remove the hard minerals but retain the alkalinity in the water.

The issue arises because alkalinity impacts the pH and overall chemistry of the brewing process. Brewers often need to adjust the alkalinity to achieve the desired mash pH and enhance enzymatic activity. Water softened by traditional methods may have reduced hardness but retains the alkalinity, which can affect the brewing process and final beer characteristics.
Brewers often explore alternative methods for adjusting water hardness and alkalinity to achieve the desired brewing water profile. This could involve blending soft and hard water or using brewing salts and minerals to achieve the appropriate balance for brewing purposes.
According to a study published in the Slovak Journal of Food Sciences, brewing water affects the content of niacin (vitamin B3) and riboflavin (vitamin B2) in beer. Beer made from hard water contains more B3 and B2 vitamin because the yeast autolysis (cell destruction) is more pronounced in this type of water.
Quick fact:
Burtonisation or Burtonising is the process of adding mineral salts to the water to recreate the water composition found in Burton-upon-Trent, a town in the Midlands known for its Pale Ale and Bitter beers. Brewers undertake this process to mimic Burton-upon-Trent’s distinctive water profile to achieve their beer’s desired characteristics.
The Water Report
Determining the alkalinity and hardness of your water can be done by referencing your city water report. Water reports typically focus on testing for contaminants, but they also provide information on Total Alkalinity and Total Hardness, which are important for brewers.
When reviewing the water report, look for the section related to Secondary Standards or Aesthetic Standards. In that section, you should find the measurements for Total Alkalinity and Total Hardness.

As a brewer, it is preferable to have Total Alkalinity below 100 ppm and ideally below 50 ppm. However, it is uncommon to find such low levels in municipal water supplies. Typically, you will see Total Alkalinity numbers ranging from 50 to 150 ppm.
By referring to the water report, you can gain valuable insights into the alkalinity and hardness of your water, allowing you to make informed decisions when it comes to water treatment and adjustments for your brewing process.
The Residual Alkalinity (RA) of water, which is primarily determined by water hardness, plays a significant role in the brewing process. The RA value indicates the water’s ability to buffer against the acidity of darker malts.
When brewing with darker malts, which have a lower pH, water with a higher RA can effectively counteract the acidity and maintain the desired pH range during the mash. If the water’s RA is not sufficiently high to handle the acidity of the darker malts, the mash pH can drop significantly. This can lead to reduced sugar extraction from the grains and result in a thin and acidic wort.
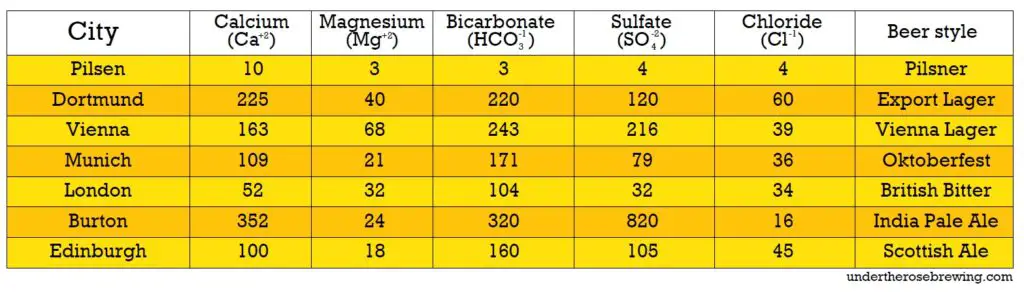
The historical examples of cities like Dublin (Ireland) and Pilsen (Czech Republic) further illustrate the impact of water characteristics on beer styles. Dublin’s water, with higher hardness and a higher RA, was well-suited to handle the acidity of darker malts. This allowed for the development of stout and other darker beer styles. On the other hand, Pilsen’s water, which is low in minerals and hardness, was unsuitable for effectively working with dark malts. As a result, Pilsen brewers focused on lighter malts and developed styles like the iconic Pilsner.
Understanding the water’s RA and its impact on pH is crucial for brewers to achieve the desired flavor profiles and characteristics in their beer. It showcases the influence of water composition on beer style development and highlights the importance of considering water chemistry as an essential aspect of the brewing process.
References:
- Punčochářová L., Pořízka J., Diviš P., Štursa V., Potravinarstvo Slovak Journal of Food Sciences, vol. 13, 2019, no. 1, p. XX-XX, doi: https://doi.org/10.5219/1046 and https://www.researchgate.net/publication/334095954_Study_of_the_influence_of_brewing_water_on_selected_analytes_in_beer (accessed July 2023)

I am a young architect with a passion that goes beyond blueprints… it’s beer! undertherosebrewing.com is more than just a blog, it’s a manifestation of my lifelong dream to explore, read, and learn everything about beer. Join the blog on this unfiltered and genuine adventure into the heart of beer culture. Cheers!







